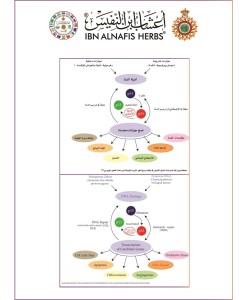
Medical Study About The Mixture CA-B11 About Cancer Treatment

VISCUM ALBUM PLANT
The mistletoe lectins in the herb are hypotensive,anti cytotoxic , and immune – stimulating . It causes improvement symptoms of chronic joint conditions, and significant lengthening of several times of cancer patients as well an improvement of quality of life.
Indications and usage:
The stem of the herb is used for its calming effect: in the treatment of mental and exhaustation: and as tranquilizer against nervous conditions such as agitation, anxiety, and increase excitability.
Approved by commission E:
TUMOR THERAPY
RHEUMATAISM
It is used for treating degenerative inflammation of joints and as palliative therapy for malignant tumors through non specific stimulation. Other uses include long term therapy for cases of mild high pressure and as atherosclerosis prophylactic.
It also may used for high blood pressure , epilepsy , whooping cough , asthma , vertiginous attack , amenorrhea , diarrhea , chorea , nervous tachycardia and hysteria.
The herb is used for joint pain, tendon and muscle pain, lumbago, back pain, vaginal bleeding during pregnancy and a Galatia.
Contraindications:
Protein oversensitivity, chronic progressive infections e.g., tuberculosis, and conditions of high fever
Precautions and adverse reactions:
No health hazards or side effects are known in conjunction with the proper administration of designated therapeutic dosages. The berries are said to have emetic and evacuant effects and to have caused the death of children. However unambiguous proof for these effects does not exist.
The wheal formation and the elevation of body temperature are considered as signs of immune system stimulation and therefore as positive therapeutic effects.
Daily dosage:
The recommended dose daily dosage is 10 gm
The dosage foe medicinal tea is 1 to 2 cups daily, EUROPEAN MISLETOE WINE dosage is 3 to 4 glasses daily.
The mechanism of the viscum album plant action as anti cancer by the reactivation of the inactivated p53 gene but what that means the researchers has approved that the gene p 53 which is located on the shot arm of the chromosome no: 17 it is an inhibitor gene for tumor growth and it is doing its role as anti malignant tumors through a lot of many mechanism play a role in apoptosis, genomic stability, and inhibition of angiogenesis through several mechanism:
1-It can activate DNA repair proteins when DNA has sustained damage
2-It can induce growth arrest by holding the cell cycle at the G/S regulation point on DNA damage recognition
3- it holds the cell for long enough , the DNA repair proteins will have time to fix the damage and the cell will be allowed to continue the cell cycle)).
It can initiate apoptosis, the programmed cell death, if DNA proves to be irreparable 4-
When the human body exposed to hypoxia or stress injury the human body is going to activate the p 53 gene to stop the cell grow thing in the g1/s phase that give the time to proteins to repair DNA so it doesn’t permit it to pass into the next stage of the cell division stages.
If there is a mutation of one of the two inherited genes the persons will susceptible to cancer occurrence an usually it develops in the two life peaks and the mutation in these genes was found in most viewed malignant tumors.
The viscum album plant fights cancers and tumors by inhibiting the growth of cancer and by stimulating a host mediated response. NATURAL KILLER cells are also promoted enhance the immune system .It is often used in conjunction with chemotherapy to increase cancer survival rates. The viscum album plant fights is being proposed as an inhibitor of HIV replication based on in vitro study.
The viscum album plant fights has also demonstrated itself to activate interferon production .VISCUM ALBUM PLANT has also been found to regenerate damaged bone marrow, increase energy levels and offer pain relief in cancer patients.
MENTHA PIPERITA :
IT IS USEFUL IN THE TREATMENT OF COMMON COLD, COUGH, BRONCHITIS, FEVER AND COLDS, INFLAMATION OF THE MOUTH AND PHARYNX, LIVER AND GALL BLADDER COMPLAINTS, DYSPEPTIC COMPLAINTS, TENDENCY TO INFECTION, anemia ,thrombocytopenia , platelet dysfunction Page number 580 in the PDR FOR HERBAL MEDICINE
In a conclusion the viscum album plant useful in the treatment of the malignant tumors in general, but after the recent experiences by the medical researchers in the company labs in CANADA it has been discovered that the adding of the MINTA PIPRETA making the mixture more powerful and useful in the treatment of malignant tumors in general and hematological based origin as a special condition
CA B11 the is a list of the malignant hematological and malignant tumors that can be treated by the mixture CA B 11
AML ACUTE MYELOID LEUKEMIA
CML CHRONIC MYELOID LEUKEMIA
LEUKEMIA ALL ACUTE LYMPHOCYTIC
CLL CHRONIC LYMPHOCYTIC LEUKEMIA
T BURKITS LYMPHOMA
HODJKIN AND NON HODJKIN
Waldenstrom’s Macroglobulinemia
Multiple Myeloma
Hairy Cell Leukemia
Sarcomas
CA B11 the is a list of the malignant gastrointestinal malignant tumors that can be treated by the mixture CA B 11
Tumors: digestive system neoplasia
GI tract
Upper GI tract
Esophagus
Squamous cell carcinoma • Adenocarcinoma
Stomach
Gastric carcinoma • Signet ring cell carcinoma • Gastric lymphoma (MALT lymphoma) • Linitis plastica
Lower GI tract
Small intestine
Duodenal cancer (Adenocarcinoma)
Appendix
Carcinoid • Pseudomyxoma peritonei
Colon/rectum
colorectal polyp: Peutz-Jeghers syndrome • Juvenile polyposis syndrome • Familial adenomatous polyposis/Gardner’s syndrome • Cronkhite–Canada syndrome
neoplasm: Adenocarcinoma • Familial adenomatous polyposis • Hereditary nonpolyposis colorectal cancer
Anus
Squamous cell carcinoma
Upper and/or lower Gastrointestinal stromal tumor • Krukenberg tumor (metastatic)
Accessory
Liver
malignant: Hepatocellular carcinoma (Fibrolamellar) • Hepatoblastoma
benign: Hepatocellular adenoma • Cavernous hemangioma
hyperplasia: Focal nodular hyperplasia • Nodular regenerative hyperplasia
Biliary tract
bile duct: Cholangiocarcinoma • Klatskin tumor
gallbladder: Gallbladder cancer
Pancreas
exocrine pancreas: Adenocarcinoma • Pancreatic ductal carcinoma
cystic neoplasms: Serous microcystic adenoma • Intraductal papillary mucinous neoplasm • Mucinous cystic neoplasm • Solid pseudopapillary neoplasm
Pancreatoblastoma
Peritoneum
Primary peritoneal carcinoma • Peritoneal mesothelioma • Desmoplastic small round cell tumor
REFERENCES
AGRAWAL, V. and SARDAR, P.R. In vitro propagation
of Cassia angustifolia through leaflet and cotyledon
derived calli. Biologia Plantarum, March 2006, vol. 50, no.
1, p. 118-122.
ARAI, M.; SAITO, T.; KANEKO, Y. and MATSUSHIMA,
H. Cellular origin and ultrastructural changes of
regenerating shoots from tobacco (Nicotiana tabacum) Beegum, A.S. et al.
121
internodes cultured in vitro. Physiologia Plantarum, April
1997, vol. 99, no. 4, p. 523-528.
BECKER, Y. and OLSHEVSKY, U. Inhibition of herpes
simplex virus replication by camptothecin. Israel Journal of
Medical Sciences, 1973, vol. 9, no. 11-12, p. 1578-1581.
BODLEY, Annette L.; CUMMING, Jared N. and
SHAPIRO, Theresa A. Effects of camptothecin, a
topoisomerase I inhibitor, on Plasmodium falciparum.
Biochemical Pharmacology, March 1998, vol. 55, no. 5, p.
709-711.
CREEMERS-MOLENAAR, J.; HAKKERT, J.C.; VAN
STAVEREN, M.J. and GILISSEN, L.J.W. Histology of the
morphogenetic response in thin cell layer explants from
vegetative tobacco plants. Annals of Botany, May 1994,
vol. 73, no. 5, p. 547-555.
DE PAIVA NETO, Vespasiano Borges; DA MOTA, Tiago
Ribeiro and OTONI, Wagner Campos. Direct
organogenesis from hypocotyl-derived explants of annatto
(Bixa orellana). Plant Cell, Tissue and Organ Culture,
November 2003, vol. 75, no. 2, p. 159-167.
DENNIS, Thomas T. and BOBAN, Philip. Thidiazuroninduced high-frequency shoot organogenesis from leafderived callus of a medicinal climber, Tylophora indica
(Furm. f.) Merrill. In Vitro Cellular and Development
Biology – Plant, March-April 2005, vol. 41, no. 2, p. 124-
128.
DHAR, Uppeandra and JOSHI, Mitali. Efficient plant
regeneration protocol through callus for Saussurea
obvallata (DC.) Edgew. (Asteraceae): effect of explant
type, age and plant growth regulators. Plant Cell Reports,
June 2005, vol. 24, no. 4, p. 195-200.
FAISAL, M. and ANIS, M. An efficient in vitro method for
mass propagation of Tylophora indica. Biologia Plantarum,
June 2005, vol. 49, no. 2, p. 257-260.
GIOVANELLA, B.C.; STEHLIN, J.S.; WALL, M.E.;
WANI, M.C.; NICHOLAS, A.W.; LIU, L.F.; SILBER, R.
and POTMESIL, M. DNA topoisomerase I-targeted
chemotherapy of human colon cancer in xenografts.
Science, November 1989, vol. 246, no. 4933, p. 1046-1048.
JAPAN PHARMACEUTICAL INFORMATION
CENTER. Drugs in Japan, Ethical Drugs. Yakugyo Jiho
Co.; Tokyo, 1995, 169-270 p.
JOSE, B. and SATHEESHKUMAR, K. In vitro mass
multiplication of Ophiorrhiza mungo Linn. Indian Journal
of Experimental Biology, June 2004, vol. 42, no. 6, p. 639-
642.
KAWIAK, Anna; KROLICKA, Aleksandra and
LOJKOWSKA, Ewa. Direct regeneration of Drosera from
leaf explants and shoot tips. Plant Cell, Tissue and Organ
Culture, November 2003, vol. 75, no. 2, p. 175-178.
KIRTIKAR, K.R. and BASU, B.D. Indian Medicinal
Plants Vol. II. 2
nd
ed. M/S Bishen Singh Mahendrapal
Singh, New Delhi, India, 1975. p. 1268-1269.
KNOLL, K.A.; SHORT, K.C.; CURTIS, I.S.; POWER,
J.B. and DAVEY, M.R. Shoot regeneration from cultured
root explants of spinach (Spinacia oleracea L.): a system
for Agrobacterium transformation. Plant Cell Reports,
December 1997, vol. 17, no. 2, p. 96-101.
LIAO, Zhihua; CHEN, Min; TAN, Feng; SUN, Xiaofen
and TANG, Kexuan. Micropropagation of endangered
Chinese aloe. Plant Cell, Tissue and Organ Culture,
January 2004, vol. 76, no. 1, p. 83-86.
LIU, Z. and LI, Z. Micropropagation of Camptotheca
acuminata decaisne from axillary buds, shoot tips, and seed
embryos in a tissue culture system. In Vitro Cellular and
Development Biology – Plant, January 2001, vol. 37, no. 1,
p. 84-88.
LO, K.H.; GILES, K.L. and SAWHNEY, V.K. Histological
changes associated with acquisition of competence for
shoot regeneration in leaf discs of Saintpaulia ionantha X
confusa hybrid (African violet) cultured in vitro. Plant Cell
Reports, March 1997, vol. 16, no. 6, p. 421-425.
LORENCE, Argelia and NESSLER, Craig L.
Camptothecin, over four decades of surprising findings.
Phytochemistry, October 2004, vol. 65, no. 20, p. 2735-
2749.
LORENCE, Argelia; MEDINA-BOLIVAR, F. and
NESSLER, Craig L. Camptothecin and 10-
hydroxycamptothecin from Camptotheca acuminata hairy
roots. Plant Cell Reports, January 2004, vol. 22, no. 6, p.
437-441.
MARTIN, K.P. Rapid in vitro multiplication and ex vitro
rooting of Rotula aquatica Lour., a rare rhoeophytic woody
medicinal plant. Plant Cell Reports, January 2003a, vol. 21,
no. 5, p. 415-420.
MARTIN, K.P. Rapid axillary bud proliferation and ex
vitro rooting of Eupatorium triplinerve. Biologia
Plantarum, December 2003b, vol. 47, no. 4, p. 589-591.
MARTIN, K.P.; JOSEPH, D.; MADASSERY, J. and
PHILIP, V.J. Direct shoot regeneration from lamina
explants of two commercial cut flower cultivars of
Anthurium andraeanum Hort. In Vitro Cellular and
Development Biology – Plant, September-October 2003,
vol. 39, no. 5, p. 500-504.
MARTIN, K.P.; SUNANDAKUMARI, C.; CHITHRA, M.
and MADHUSOODANAN, P.V. Influence of auxins in
direct in vitro morphogenesis of Euphorbia nivulia, a Rapid propagation of camptothecin producing plant, Ophiorrhiza prostrata
122
lectinacious medicinal plant. In Vitro Cellular and
Development Biology – Plant, May-June 2005, vol. 41, no.
3, p. 314-319.
MURASHIGE, T. and SKOOG, F. A revised medium for
rapid growth and bioassays with tobacco tissue cultures.
Physiologia Plantarum, October 1962, vol. 15, no. 43, p.
473-497.
MURASHIGE, T. Plant propagation through tissue
cultures. Annual Review of Plant Physiology, June 1974,
vol. 25, p.135-166.
PANTAZIS, Panayotis; HAN, Zhiyong; CHATTERJEE,
Devasis and WYCHE, James. Water-insoluble
camptothecin analogues as potential antiviral drugs.
Journal of Biomedical Science, January 1999, vol. 6, no. 1,
p. 1-7.
PRIEL, E.; SHOWALTER, S.D. and BLAIR, D.G.
Inhibition of human immunodeficiency virus (HIV-1)
replication in vitro by noncytotoxic doses of camptothecin,
a topoisomerase I inhibitor. AIDS Research and Human
Retroviruses, 1991, vol. 7, no. 1, p. 65-72.
PRUSKI, Kris; ASTATKIE, Tess and NOWAK, Jerzy.
Tissue culture propagation of Mongolian cherry (Prunus
fruticosa) and Nanking cherry (Prunus tomentosa). Plant
Cell, Tissue and Organ Culture, August 2005, vol. 82, no.
2, p. 207-211.
RAJASEKHARAN, K.; HEIN, M.B.; DAVIS, G.C.;
CARNES, M.G. and VASIL, I.K. Endogenous growth
regulators in leaves and tissue cultures of Pennisetum
purpureum Schum. Journal of Plant Physiology, 1987, vol.
130, no. 1, p.13-25.
REDINBO, Matthew R.; STYEWART, Lance; KUHN,
Peter; CHAMPOUX, James J. and HOL, Wim G.J. Crystal
structures of human topoisomerase I in covalent and monocovalent complexes with DNA. Science, March 1998, vol.
279, no. 5356, p. 1504-1513.
ROJA, G. Comparative studies on the camptothecin content
from Nothapodytes foetida and Ophiorrhiza species.
Natural Product Research, January 2006, vol. 20, no. 1, p.
85-88.
ROUT, Gyana Ranjan. Direct plant regeneration of curry
leaf tree (Murraya koenigii Koenig.), an aromatic plant. In
Vitro Cellular and Development Biology – Plant, MarchApril 2005, vol. 41, no. 2, p. 133-136.
SAITO, K.; SUDO, H.; YAMAZAKI, M.; KOSEKINAKAMURA, M.; KITAJIMA, M.; TAKAYAMA, H. and
AIMI, N. Feasible production of camptothecin by hairy root
cultures of Ophiorrhiza pumila. Plant Cell Reports, March
2001, vol. 20, no. 3, p. 267-271.
SHRIVASTAVA, N. and RAJANI, M. Multiple shoot
regeneration and tissue culture studies on Bacopa monnieri
(L.) Pennell. Plant Cell Reports, August 1999, vol. 18, no.
11, p. 919-923.
SHU, Q.Y.; LIU, G.S.; QI, D.M.; CHU, C.C.; LIU, J. and
LI, H.J. An effective method for axillary bud culture and
RAPD analysis of cloned plants in tetraploid black locust.
Plant Cell Reports, October 2003, vol. 22, no. 3, p. 175-
180.
SIVARAM, Latha and MUKUNDAN, Usha. In vitro
culture studies on Stevia rebaudiana. In Vitro Cellular and
Development Biology – Plant, September-October 2003,
vol. 39, no. 5, p. 520-523.
ŠTAJNER, Nataša; BOHANEC, Borut and JAKŠE,
Marijana. In vitro propagation of Asparagus maritimus – A
rare Mediterranean salt-resistant species. Plant Cell, Tissue
and Organ Culture, September 2002, vol. 70, no. 3, p. 269-
274.
SUDO, Hiroshi; YAMAKAWA, Takashi; YAMAZAKI,
Mami; AIMI, Norio and SAITO, Kazuki. Bioreactor
production of camptothecin by hairy root cultures of
Ophiorrhiza pumila. Biotechnology Letters, March 2002,
vol. 24, no. 5, p. 359-363.
SUNANDAKUMARI, C.; ZHANG, C.-L.; MARTIN, K.P.;
SLATER, A. and MADHUSOODANAN, P.V. Effect of
auxins on indirect in vitro morphogenesis and expression of
gusA transgene in a lectinacious medicinal plant, Euphorbia
nivulia Buch.-Ham. In Vitro Cellular and Development
Biology – Plant, September-October 2005, vol. 41, no. 5, p.
695-699.
TAFUR, S.; NELSON, J.D.; DELONG, D.C. and
SVOBODA, G.H. Antiviral components of Ophiorrhiza
mungos isolation of camptothecin and 10-
methoxycamptothecin. Lloydia, 1976, vol. 39, no. 4, p. 261-
262.
TAKEUCHI, S.; DOBASHI, K.; FUJIMOTO, S.;
TANAKA, K.; SUZUKI, M.; TERASHIMA, Y.;
HASUMI, K.; AKIYA, K.; NEGISHI, Y. and TAMAYA,
T. A late phase II study of CPT-11 in gynecologic cancers.
Research groups of CPT-11 in gynecologic cancers.
Japanese Journal of Cancer and Chemotherapy, August
1991, vol. 18, no. 10, p. 1681-1689.
TIWARI, V.; SINGH, B.D. and TIWARI, K. N. Shoot
regeneration and somatic embryogenesis from different
explants of Brahmi [Bacopa monniera (L.) Wettst.]. Plant
Cell Reports, April 1998, vol. 17, no. 6-7, p. 538-543.
TWYFORD, C.T. and MANTELL, S.H. Production of
somatic embryos and plantlets from root cells of the greater
yam. Plant Cell, Tissue and Organ Culture, July 1996, vol.
46, no. 1, p. 17-26.Beegum, A.S. et al.
123
WALL, M.E.; WANI, M.C.; COOK, C.E.; PALMER,
K.H.; MCPHAIL, A.T. and SIM, G.A. Plant anti-tumor
agents: I. The isolation and structure of camptothecin, a
novel alkaloidal leukemia and tumor inhibitor from
Camptotheca acuminata. Journal of the American
Chemical Society, August 1966, vol. 88, no. 16, p. 3888-
3890.
WATASE, I.; SUDO, H.; YAMAZAKI, M. and SAITO, K.
Regeneration of transformed Ophiorrhiza pumila plants
producing camptothecin. Plant Biotechnology, 2004, vol.
21, no. 5, p. 337-342.
WELANDER, M. Plant regeneration from leaf and stem
segments of shoots raised in vitro from mature apple trees.
Journal of Plant Physiology, 1988, vol. 132, no. 6, p. 738-
744.
WENZEL, C.L. and BROWN, D.C.W. Histological events
leads to somatic embryo formation in cultured petioles of
alfalfa. In Vitro Cellular and Development Biology – Plant,
1991, vol. 27, p. 190-196.
ZHANG, Chun-Lai; CHEN, Dong-Fang; ELLIOTT,
Malcolm C. and SLATER, Adrian. Thidiazuron-induced
organogenesis and somatic embryogenesis in sugar beet
(Beta vulgaris L). In Vitro Cellular and Development
Biology – Plant, March 2001, vol. 37, no. 2, p. 305-310